Coronavirus (COVID-19) Updates
For the latest COVID-19 information and updates from Qatar Foundation, please visit our Statements page
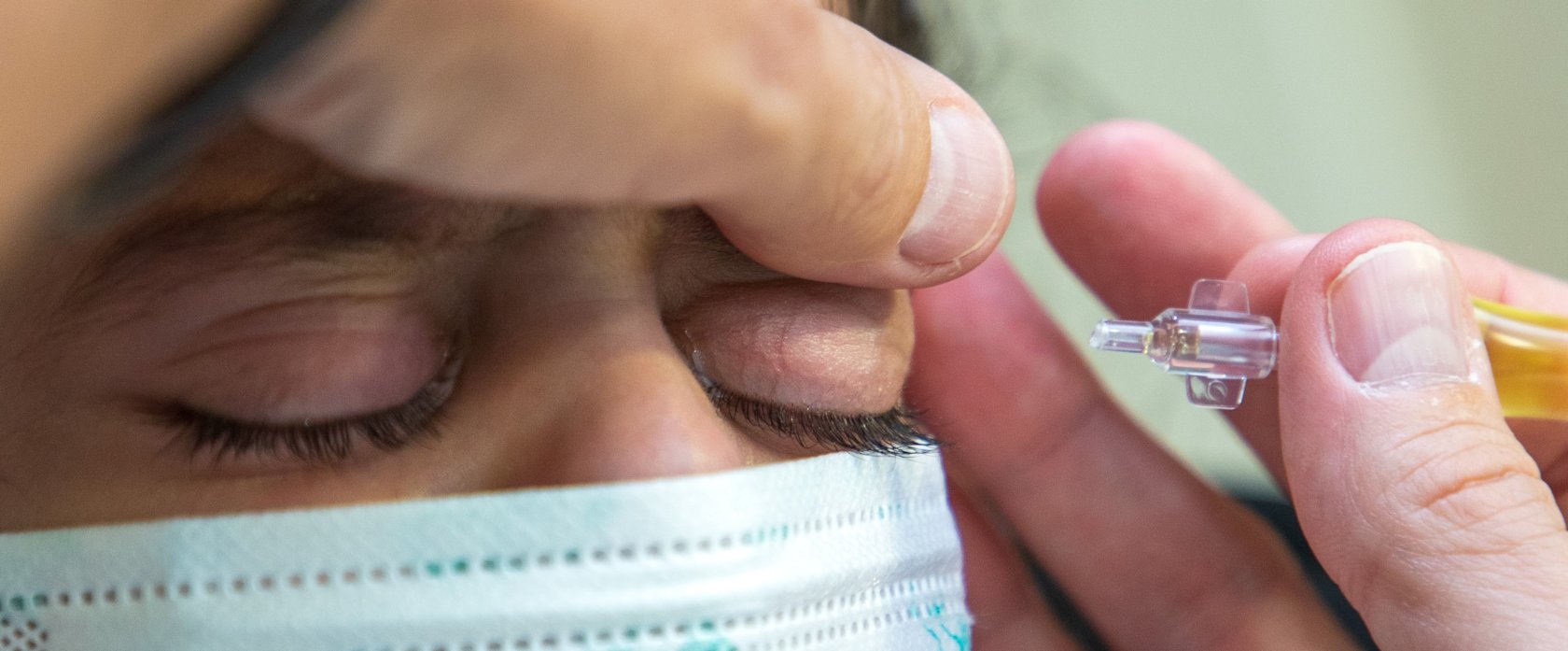
Multidisciplinary team effort at Sidra Medicine produces country’s first plasma eye drops, treats Qatari baby with “one-in-a-million” condition
In early 2020, the family of a 10-month old Qatari child suffering from an ultra-rare disease called plasminogen deficiency, that was causing the child’s eyes to fill with woody growths, got in touch with Sidra Medicine, a member of Qatar Foundation. The family, then in the UK, was desperate to return to Qatar given the rising number of COVID-19 cases in the UK, but were afraid that their daughter would not receive the right treatment back home.
However, doctors at Sidra Medicine, the only hospital in Qatar that offers precision medicine to pediatric patients, were positive that it could offer treatment for this condition. Dr. Chiara Cugno, Director of the Advanced Cell Therapy Core at Sidra Medicine, said: “When we were contacted about this case, we were confident right from the beginning that we had the expertise available to do this.
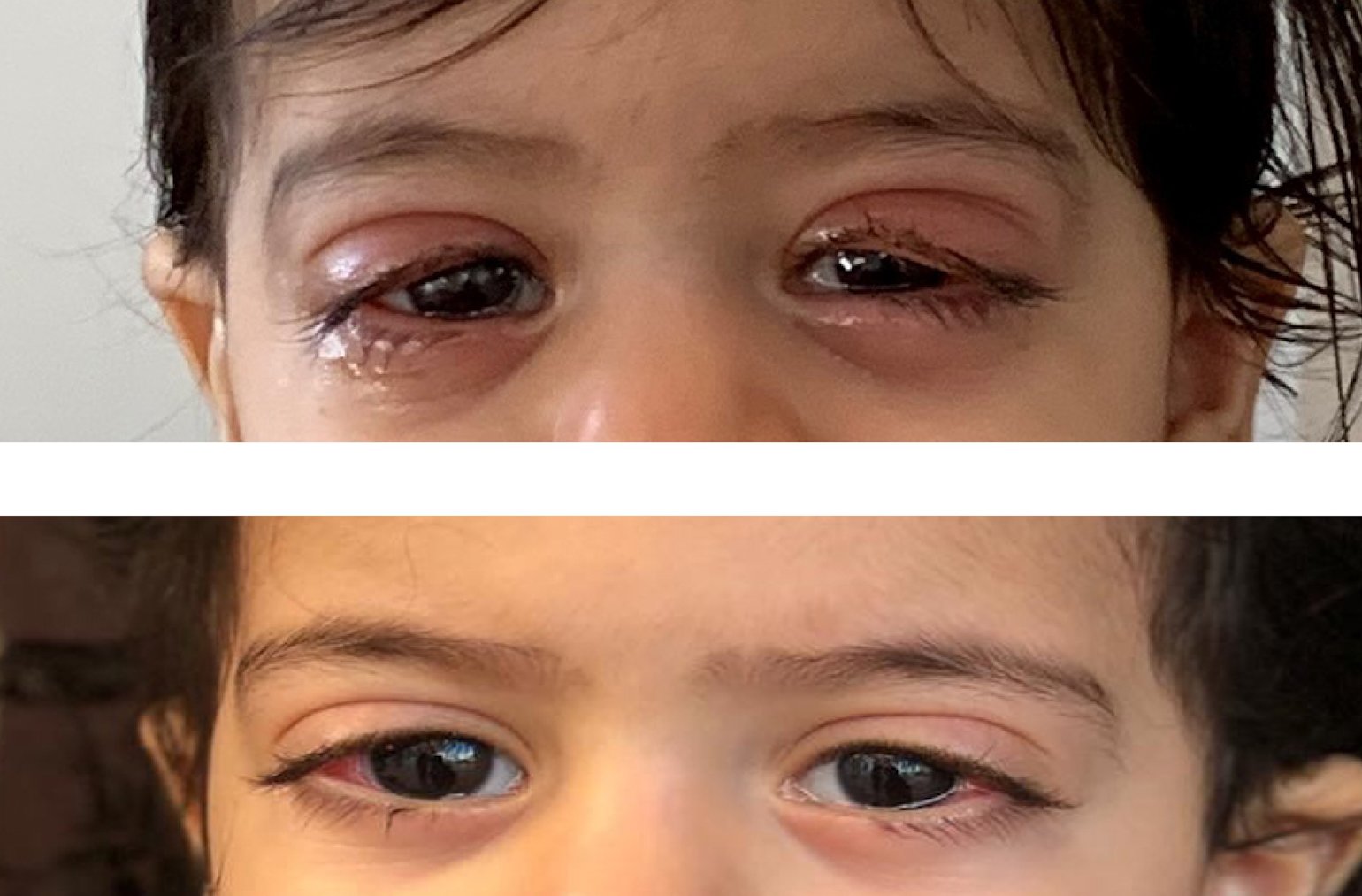
Image of the child’s eyes before treatment (top) and after treatment (bottom)
“The only thing was that the treatment – plasma eye drops – had never been produced in Qatar before, not just at Sidra Medicine, but anywhere else in the country. So, we understood that regulatory approvals would take a while, but since we already had a strong foundation to develop the eye drops in-house, we pooled in all our expertise to help this young patient.”
There was a time my daughter couldn’t open her eyes; I was terrified she was going to lose her eyesight.
The child’s mother said: “There was a time my daughter couldn’t open her eyes; I was terrified she was going to lose her eyesight.”
The prevalence of plasminogen deficiency is estimated at 1.6 per one million people. A personalized approach, using eye drops, is the only option currently available for managing this rare disease, which is also classed as an orphan disease – a disease with no cure. To manage the disease, the patient must use eye drops containing plasmin – the enzyme that the patient is lacking. The eye drops are made using the plasma of a donor who is a match for the patient.
The woody growths caused by plasminogen deficiency can be surgically removed, but to stop them from growing back, the patient needs special eye drops made from human plasma or serum containing plasminogen
“The woody growths caused by plasminogen deficiency can be surgically removed, but to stop them from growing back, the patient needs special eye drops made from human plasma or serum containing plasminogen,” said Dr. Pedro Mattar, Senior Attending Physician, Ophthalmology Division at Sidra Medicine.
While the family did initially import the eye drops from the UK, the team at Sidra Medicine was adamant at producing these eye drops locally. Because the eye drops are not a one-time treatment and need to be administered every few hours, the family would continue needing them in the future, and if they aren’t available locally, the family would either have to travel to the UK every three months or import them.
Dr. Cugno said: “Neither of these choices is sustainable or economical. There was no choice but for us to do it here in Qatar.”
The team working on producing the eye drops consisted of members from various departments across Sidra Medicine including Pediatric Hematology, Ophthalmology, Pathology Blood Bank and Advanced Cell Therapy Core. To speed up the process, instead of locating a donor and then screening them to ensure they are healthy, the team decided to use existing fresh frozen plasma from screened donors that is usually used for infusion.
“Turning the donated blood plasma into eyedrops not only required a great deal of planning but also careful and extensive review of available literature to guarantee that Sidra Medicine followed the highest global quality assurance protocols,” said Dr. Eileen McBride, Medical Director of the Transfusion Medicine laboratory at Sidra Medicine.
“Because this was the first time something like this was being done, we found ourselves looking for an automated sterile production system which could fill several eye drop vials to be given to the family for use over three months. Due to the pandemic, each step of the process was delayed, but thanks to the determination of all the teams, we eventually succeeded,” said Massimino Jan Miele, Manager of Advanced Cell Therapy Core Laboratories at Sidra Medicine.
The child’s mother said she often worried if the doctors and researchers at Sidra Medicine would be able to channel enough of their time and commitment towards her daughters’ case, given she was the only one in the entire country with this disease.
“It seemed like maybe the effort was too much to put in into one patient. Yet they still did it, and with so much zeal. Despite the pandemic my daughter’s case was never put on the back burner and remained a top priority,” she said.
The fact that we were able to produce these eye drops on our own without relying on centers abroad, is a great example of how Qatar as a country has truly embarked on its journey to delivering personalized medicine and becoming self-sufficient in medicine.
In May 2021, the family received the locally produced eye drops for their child. Dr. Ayman Saleh, Chief of Pediatric Oncology and Hematology at Sidra Medicine said: “This was a truly joint effort, not just between the different teams at Sidra Medicine but also the family. We are grateful to the parents for trusting us and allowing us the opportunity to produce these eye drops. This project was not only a first for Sidra Medicine, but also a first for Qatar.”
The child’s mother explained that previously the responsibility of arranging the transport of the eyedrops from the UK to Qatar was entirely on her and her husband. The whole process was extremely difficult for them, not just emotionally but also physically.
The eye drops are very sensitive to temperature and must be kept frozen while being transported. It is a high-risk process with multiple links; one failure and the whole shipment can be wasted.
The child’s mother said: “With the eye drops now being produced at Sidra Medicine, we have been relieved of that massive responsibility. Having a top-notch hospital like Sidra Medicine tell us that they will make the medicine, store it, and ensure we have enough supply, makes me feel like we can sleep again, and that once again we have the time and energy for other things in life.”
Despite the regulatory hurdles and the COVID-related delays, the team is pleased they were eventually able to provide the child with the treatment. Dr. Cugno said: “The fact that we were able to produce these eye drops on our own without relying on centers abroad, is a great example of how Qatar as a country has truly embarked on its journey to delivering personalized medicine and becoming self-sufficient in medicine.
“This experience has paved the way for us to include other tools in the therapeutic resources that we have developed for kids in Qatar, without the need to travel or rely on expensive treatment coming from overseas.”











